Primary and secondary batteries are electrochemical cells that convert stored chemical energy to electrical energy.
What are batteries?
So, what is a battery? A battery is an electrochemical cell or cells that produce an electric current by converting stored chemical energy to electrical energy.
Because of their ability to store energy, primary and secondary batteries are vital to modern-day electronic products such as mobile phones, tablets, smartwatches, laptops, E-scooters, bicycles, and drones. For battery-powered products, one of the critical decisions product designers must make early in the product design process is what type of battery to use for the new electronic product.

There are numerous battery types with various characteristics for new product development. Because each device necessitates a unique battery to meet the power supply requirements, it is critical to understand the product requirements, such as voltage, peak current, operating environment, temperature rating, and life span, when selecting the battery type.
The following sections discuss the battery types, their chemical composition, applications, and the critical parameters for choosing the suitable battery for your next electronic device.
Primary and Secondary batteries
There are two basic types of batteries: primary and secondary. These batteries power most portable consumer electronics products as they have many of the same characteristics and functions.
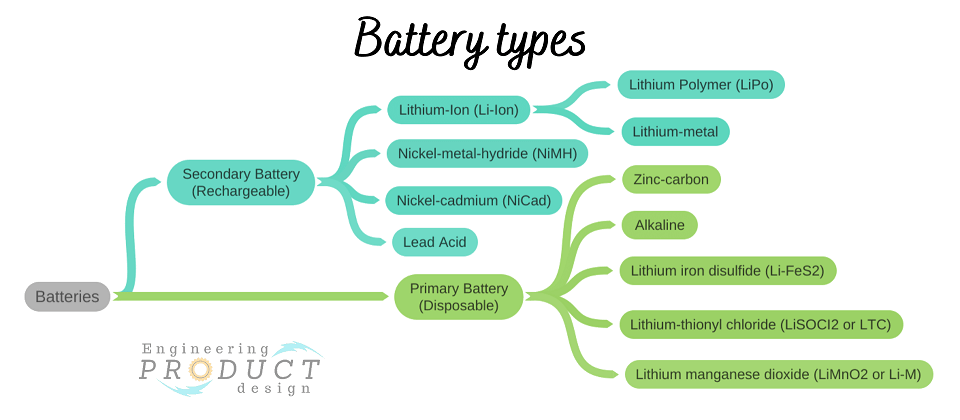
The image above shows the widely used battery types for both primary and secondary.
What are primary batteries and secondary batteries?
The primary batteries, also known as Disposable batteries, are non-rechargeable; therefore, the user can only use them once. The initial charge of a disposable battery can last significantly longer than a rechargeable battery in most applications. Their design is simple and lighter, and there is no fluid used in these batteries hence called Dry cells such as Zinc-Carbon cells.
However, the secondary batteries are rechargeable and have a longer life span because the user can recharge them multiple times. Their complex design consists of diverse materials for their anode, cathode and electrolytes. Lead-acid, nickel-cadmium (NiCd), and lithium-ion batteries are examples of secondary batteries.
| Primary battery | Secondary battery |
| Low initial cost | High initial cost |
| They are comparably smaller and lighter | They are complex and heavier |
| Have high energy density | Have a smaller energy density |
| High internal resistance | Low internal resistance |
| Irreversible chemical reaction | Reversible chemical reaction |
| Simple and easy to use | Need special circuits to protect |
| Slow discharge | Faster discharge |
| Cells don’t have any fluid | Cells contain liquid chemicals |
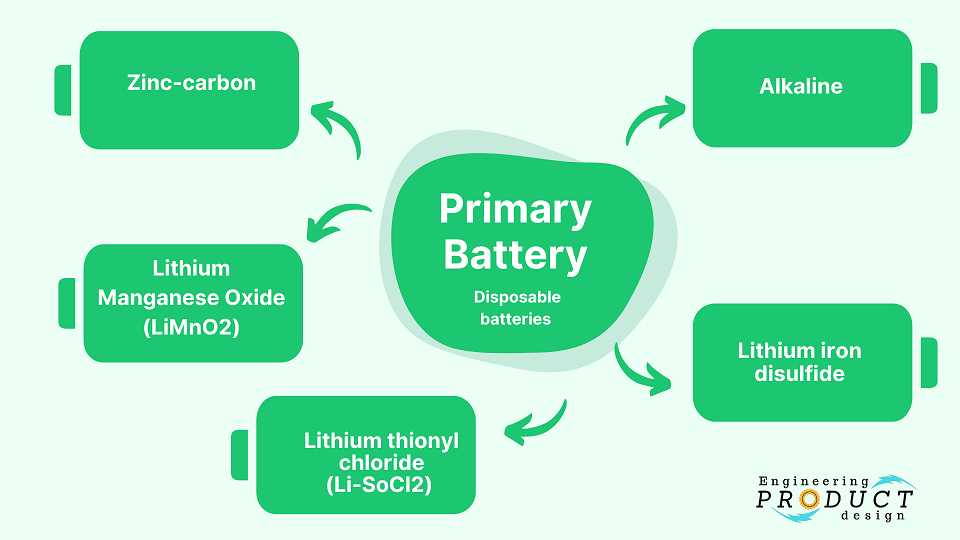
Primary Battery
Primary batteries, also known as disposable batteries, are designed for single use as the electrochemical reaction is not reversible. The most common primary battery types are Alkaline, Zinc Carbon, Lithium iron disulfide, Lithium-thionyl chloride, Lithium manganese dioxide, and Lithium-sulfur dioxide. These come in various standard sizes, such as D, C, AA, AAA, AAAA, 9V, and coin cells.
Zinc Carbon Battery
This battery is a primary dry cell battery. It produces current through the electrochemical reaction between the zinc anode and carbon cathode in the existence of an ammonium chloride electrolyte.

Zinc-carbon battery applications: Manufacturers use Zinc-Carbon batteries in Toys, Clocks, TV remotes and Flashlights.
Advantages of Zinc-carbon battery: Inexpensive and reliable
Disadvantages of Zinc-carbon battery: Poor leakage resistance, unsuitable for cold weather, low energy density, voltage drop steadily with discharge.
Zinc-Carbon battery design tips
- The product designer can use zinc-carbon batteries if budget is the primary concern.
- They are unsuitable if a large amount of power and sizeable current storage capacity is required.
Alkaline Battery
Alkaline battery, also known as Alkaline-manganese, is an advanced form of Zinc-carbon battery and delivers more energy at higher current loads. The battery produces power from the chemical reaction between Zinc and Manganese dioxide.
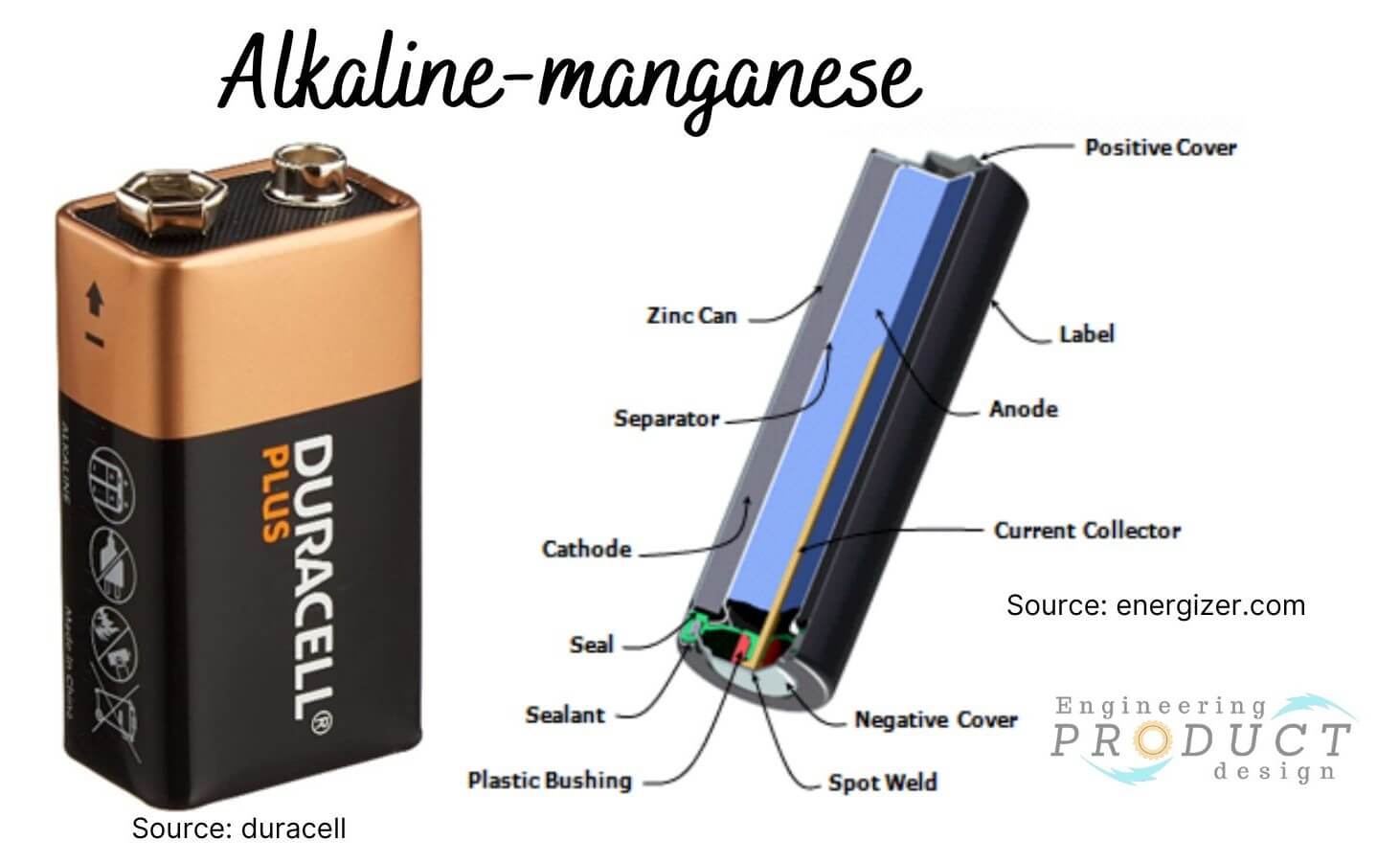
Alkaline battery applications: Many household items, including gaming consoles, remote control, CD players, digital cameras, toys, flashlights, and radios, use alkaline batteries.
Advantages of Alkaline Battery: Alkaline batteries have a low self-discharge rate and do not leak electrolytes.
Disadvantages of Alkaline Battery: Their high internal resistance reduces the battery power output.
Alkaline battery design tips
- When Alkaline batteries discharge, a small amount of hydrogen gas is released, and battery-powered devices must allow for venting.
Lithium iron disulfide (LiFeS2)
The chemistry and construction of lithium iron disulfide batteries differ from those of alkaline batteries. They use lithium as an anode, iron disulfide as a cathode, and lithium salt and organic solvent blend as electrolytes. The diagram below shows a cross-section of a typical cylindrical LiFeS2 battery.
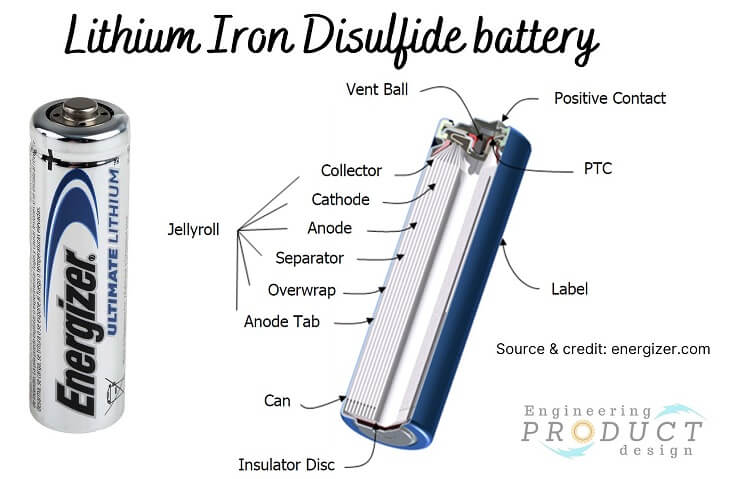
Applications: Lithium Iron batteries are used in electronic devices that require a small, portable power source, such as digital cameras, portable lights and bike lights
Advantages:
- 30% lighter than typical alkaline batteries
- -40°C to 60°C temperature range
- Excellent quality
- Longevity and durability
- Have lower resistance than Alkaline and higher capacity.
Disadvantages: Because of the lithium metal content in the anode, the Li-FeS2 has a higher price and transportation issues.
Design guide :
- Do not mix battery types from different manufacturers or chemistries
- The Li-FeS2 includes safety devices such as a positive thermal coefficient (PTC) that limits current at high temperatures and resets when temperatures return to normal.
Lithium-thionyl chloride (Li-SoCl2)
Lithium thionyl chloride is a primary cell battery. Lithium-thionyl chloride (Li-SOCl2) cells use a metallic lithium positive electrode (anode) and a liquid negative electrode (cathode) consisting of a porous carbon current collector filled with thionyl chloride.
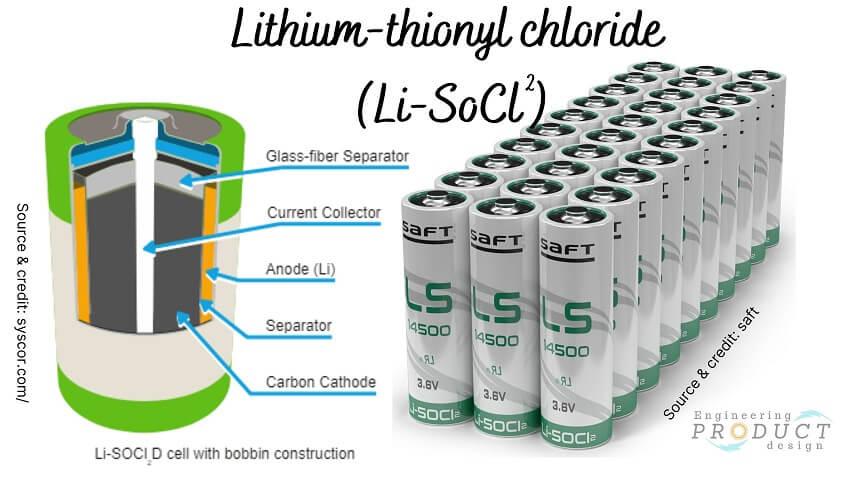
Lithium-thionyl chloride battery applications: Lithium Thionyl Chloride custom size batteries are used in electronic devices that require a small, compact power source, such as clock supports, smart sensors, system backups, real-time clocks and automotive electronics.
Lithium-thionyl chloride battery advantages
- High voltage response,
- Stable across its lifetime
- Low self-discharge rate
- Ease of use
- Wide operating temperature range (-55°C to 70°)
- Extended storage and operational life (10 years)
Lithium-thionyl chloride battery disadvantages
- Voltage delay
- Regardless of the precautions, an uncontrollable heat explosion occurs at high-temperature discharge and explodes.
- High price
- Environmental pollution during the manufacture
Lithium manganese dioxide (LiMnO2)
Lithium Manganese Oxide (LiMnO2) batteries use manganese as the cathode and lithium as the anode. LiMnO2 is available in various shapes, the most common of which are button cells and cylindrical batteries.
Applications: They are widely used in electricity, gas and water meters, fire and smoke alarms and security devices.
Advantages
- High Operational Safety (UL certified)
- High Cell Voltage (3.3V)
- A wide temperature of operation
- Low Self Discharge
- High Energy Density
- High Reliability
- Inorganic Electrolyte
- Non-Pressurized System
- Solid Cathode
Primary battery comparison
| Primary battery | Alkaline | Lithium iron disulfide (LiFeS2) | Lithium-thionyl chloride (LiSOCI2 or LTC) | Lithium manganese dioxide (LiMnO2 or Li-M) |
|---|---|---|---|---|
| Specific energy | 200Wh/kg | 300Wh/kg | 500Wh/kg | 280Wh/kg |
| Voltage | 1.5V | 1.5V | 3.6–3.9V | 3–3.3V |
| Power | Low | Moderate | Excellent | Moderate |
| Passivation | N/A | Moderate | Moderate | Moderate |
| Safety | Good | Good | Precaution | Good |
| Pricing | Low | Economical | Industrial | Economical |
| Shelf life | 10 years | 15 years | 10–20 years | 10–20 years |
| Operating temp | 0°C to 60°C | 0°C to 60°C | -55°C to 85°C higher for a short time | -30°C to 60°C Some enable from -55°C to 90°C |
Secondary Battery
As discussed in the previous section, secondary batteries are rechargeable and found in products such as mobiles, tablets, laptops, e-scooters and many more portable devices.

Lithium Ion (Li-Ion) Battery
A lithium-ion battery, also known as a Li-ion battery, is a rechargeable battery made up of cells in which lithium ions move from the cathode to the anode via an electrolyte during discharge and back again during charging.
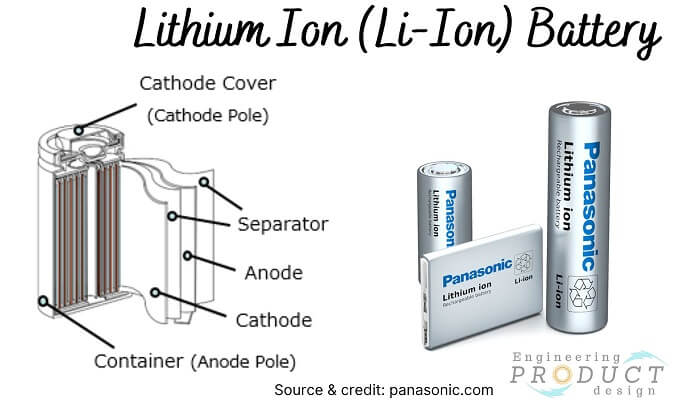
There are six types of Lithium-ion batteries depending on the active material.
- Lithium Cobalt Oxide(LiCoO2) — LCO
- Lithium Manganese Oxide (LiMn2O4) — LMO
- Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) — NMC
- Lithium Iron Phosphate(LiFePO4) — LFP
- Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) — NCA
- Lithium Titanate (Li2TiO3) — LTO
Lithium-ion cells consist of an intercalated lithium compound positive electrode, graphite negative electrode and electrolyte lithium hexafluorophosphate (LiPF6) salt dissolved in an organic solvent. As a result, lithium-ion batteries have a high energy density, no memory effect, and a low self-discharge rate.
Lithium Ion battery applications
- Power tools
- Electric vehicles
- Laptop computers
- Tablets and smartphones
- Small digital cameras
Lithium-ion rechargeable batteries can be found in every iPhone, iPad, iPod, Apple Watch, MacBook, and AirPods. Apple uses Lithium-ion batteries because they are far better than other types for their products.

Lithium Ion (Li-Ion) Battery advantages and disadvantages
Advantages: High level of energy density, Lightweight, Less maintenance, Low self-discharge rate
Disadvantages: Can be damaged due to overheating and high voltage
Lithium Ion (Li-Ion) Battery design tips
- When to use: If there is a requirement of high energy density and high discharge current.
- When not to use: If low-cost transportation, compliance testing is required.
Lithium Polymer (LiPo) Battery
The materials of the electrodes are the same as Lithium-ion batteries but use a high-conductivity gel polymer as an electrolyte to enable the movement of ions between electrodes. In addition, this polymer electrolyte can shut down the battery due to overheating during charging and discharging.
Lithium Polymer battery applications
- Laptop computers
- Tablets
- Smartphones
- lightweight electric vehicles
- aircraft
Lithium Polymer (LiPo) battery advantages and disadvantages
Advantages: Comparatively secure than Li-Ion batteries, High level of energy density, less maintenance, factors related to slim and flexible form.
Disadvantages: Expensive, unsafe during leakage or puncture.
Lithium Polymer design tips
- When to use: If there is a requirement for high energy density and low self-discharge rate.
- When not to use: Not suitable for low-temperature applications.
Nickel-Cadmium (NiCad) Battery
Nickel-Cadmium batteries are secondary type batteries that typically contain a Cadmium hydroxide anode, a Nickel oxide-hydroxide cathode, and a Potassium hydroxide electrolyte between both electrodes.
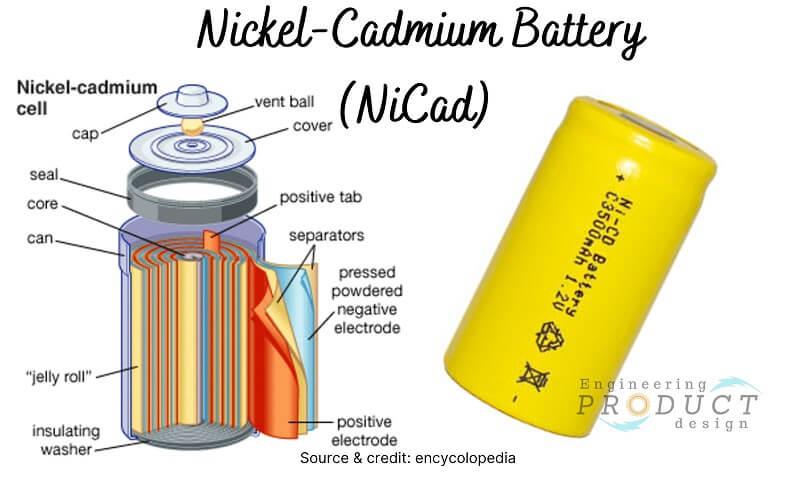
Nickel Cadmium battery applications
- Medical equipment
- Power tools
- Radios
- Handheld walkie talkies
Advantages of NiCad: fast charging, long shelf life, higher charge and discharge cycle.
Disadvantages of NiCad: Expensive, unsafe during overcharging, Cadmium is not environment friendly.
Design tips for NiCad
- In case of cheaper rechargeable battery requirements.
- If a longer life span is required for multiple times charging and discharging
- Do not use if recharging conditions are undefined.
Nickel Metal Hydride (NiMH) Battery
The composition of A Nickel-Metal Hydride (NiMH) secondary battery consists of a positive electrode of nickel oxide-hydroxide and a negative electrode with a hydrogen-absorbing alloy. The electrodes are unconnected with a potassium hydroxide electrolyte.
Nickel-Metal Hydride (NiMH) battery applications: Digital cameras, wireless telephones, microphone-based products, toothbrushes, Medical instruments, and hybrid vehicles
NiMH advantages: High power density, compact size, No transportation regulations, a good substitute for Alkaline.
NiMH disadvantages: Expensive than a NiCad battery, rapidly self-discharges, and has a short service life.
Design tips for Nickel-Metal Hydride
- Suitable to use for high current drain usage, i.e. portable power tools.
- If the battery is at half capacity and the charging method is undefined.
- NiMH battery has a shorter life span. Hence, it does not match more extended usage requirements.
Lead-acid batteries
The lead acid battery is rechargeable with a metallic lead electrode (anode), a lead dioxide electrode (cathode) and a concentrated solution of sulphuric acid (35%–40%) as an electrolyte. It is inexpensive, capable of producing high currents, and has a relatively low energy density.
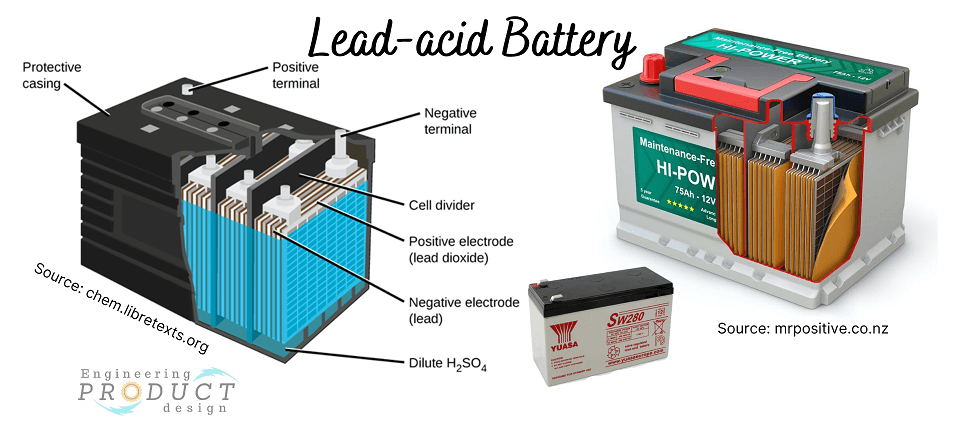
Lead-acid battery applications
- Machinery and Automobiles
- Small power storage systems
- UPS
- starting lighting and ignition power sources for automobiles
- large-scale power systems, such as grid-scale power systems
Advantages of Lead-acid: Economical, less maintenance, high-level discharge rate
Disadvantages of Lead-acid: Short service life, heavyweight, limited useable capacity
Lead-acid design tips
When to use
- For storing a large quantity of power
- If a low-cost battery is required
When not to use
- Suppose a lightweight battery is needed. Unfortunately, these batteries are heavier due to heavy lead cells.
- If there is a requirement for numerous cycles
Secondary battery comparison
| Description | NiCad | NiMH | Lead Acid | Li-Ion | Li-Polymer |
|---|---|---|---|---|---|
| Gravimetric Energy Density (Wh/kg) | 45 – 80 | 60 – 120 | 30 – 50 | 110 – 160 | 100 – 150 |
| Internal resistance (mΩ) | 100 – 200 | 200 – 300 | <100 | 150 – 250 | 200 – 300 |
| (includes peripheral circuits) | 6V pack | 6V pack | 12V pack | 7.2V pack | 7.2V pack |
| Cycle Life (to 80% of initial capacity) | 1500 | 300 – 500 | 200 – 300 | 500 – 1000 | 300 – 500 |
| Fast Charge Time | 1h typical | 2-4h | 8-16h | 2-4h | 2-4h |
| Overcharge Tolerance | moderate | low | high | very low | low |
| Self-discharge / Month (room temp) | 0.2 | 0.3 | 0.05 | 0.1 | 0.1 |
| Cell Voltage (nominal) | 1.25V | 1.25V | 2V | 3.6V | 3.6V |
| Load Current – peak | 20C | 5C | 5C | >20C | >20C |
| Load Current – best result | 1C | 0.5C or less | 0.2C | 5C or less | 5C or less |
| Operating Temperature | -40 to 60°C | -20 to 60°C | -20 to 60°C | -20 to 60°C | 0 to 60°C |
| Maintenance Requirement | 30 – 60 days | 60 – 90 days | 3 – 6 months | not req. | Not req. |
Battery selection criteria
There is no single battery design that is ideal for every application. Choosing one necessitates a trade-off. That is why it is critical to prioritise your requirements list. Determine which parameters you must have and which you can compromise. Here are some key parameters to consider during the early stages of product design.
Rechargeable or Disposable
This parameter relates to one-time or multiple-time usage. For example, the non-rechargeable battery is used in mems-based sensors, toys, smart watches, pacemakers, and flashlights. At the same time, the rechargeable battery is used in laptops, cell phones, and EVs to provide regular power.
Space availability
The batteries have different sizes and shapes, as mentioned above. However, the typical sizes of primary and secondary batteries/cells are AA, AAA, and 9V, which are feasible for portable gadgets.
Battery voltage
As mentioned in the specifications table, this parameter is described as a battery’s nominal or output voltage.
Operating temperature
The temperature also affects the battery performance. The batteries with liquid electrolytes cannot operate below 0°C as their electrolyte has a higher chance of freezing. Similarly, lithium-based batteries can perform up to -40°C but with low performance. The ideal temperature range of these batteries is 20°C to 40°C.
Battery capacity
The battery capacity is expressed as Watt-hours (Wh) which shows the amount of power (W) delivered for a specific time range. It relies on temperature, discharge rate, and cut-off voltage value. For example, a battery with 12V and 1Ah has a total capacity of 12Wh, whereas it can deliver 1 Amp for one hour or 100mA for 10 hours or 10mA for 100 hours which is called the discharge rate. The cut-off voltage value is the point at which the battery is considered fully discharged, and further discharge can be harmful.
Chemical composition
The characteristics of a battery always depend on its chemical composition, as described earlier.
Battery cost
A battery is considered among the most expensive parts of any device. Hence product designer should select it according to your budget and the need of the application.
Shelf Life
The shelf life is also essential when choosing a battery as it determines how long a battery can be kept unutilised.